The lab will be closed to visitors Monday, August 15 as our building finishes asbestos abatement. Please drop-off your sample later in the week or leave it in the cooler at the entrance of the hallway for us to collect. Thank you!
Welcome to the Team, Lou!
We are excited to announce the hiring of our new undergraduate Extension assistant: Chenghao “Lou” Lou.
Lou will help us produce elevated extension materials including videos and podcast episodes. Stay tuned for these resources!
Welcome to the team, Lou!
Hiring Undergraduate Laboratory & Extension Assistant
The UConn Plant Diagnostic Laboratory (PDL) is seeking applicants for part-time student assistants (2). One position will support the PDL with media preparation, sample receipt, client data entry, sample plating/incubation, and PDL email account management. The other assistant will support the PDL and UConn Home & Garden Education Center with occasional outreach events and extension publications, such as blog posts, newspaper articles, and fact sheets (reviewed and edited by supervisor prior to publication).
These positions will help prepare a student for work in more advanced laboratory settings, entry-level extension positions, and positions in the nursery and horticulture industry. Useful skills will be developed and extension-style publications are possible to enhance one’s CV/resume.
– Salary is $15.50 – $18.00/hr, depending on experience and qualifications (see below)
– Flexible schedule and number of hours (min 10 hrs/week; max 30hrs/week)
– Grant-funded through Aug.31st
o May be renewed on 8/31/22 depending on applicant interest and availability of grant funding
o If continuing beyond 8/31/22, positive performance may lead to raise in salary as well as opportunities to support research and attend/present at professional meetings (APS, ESA, NPDN, etc.)
– Work computer and other necessary equipment will be provided for the duration of employment
Required Qualifications:
• Basic knowledge of laboratory equipment and safety protocols
• Must have passed, with a grade of “B” or higher, Fundamentals of Plant Pathology
o Will consider any of the following as substitutions with justification from applicant: Herbaceous Ornamental Plants, Introduction to the Horticulture of Cannabis, Introduction to Plant Science, Nursery Production, Plant Pest Control, Plant Propagation, Small Fruit Production, Turfgrass Management, or Vegetable Production
• Proficient with Microsoft Office (Word, Excel, PowerPoint)
• Must be comfortable handling live plants, insects, soil, and fungi on a routine basis
• Must comply with current University masking and vaccination policy, as well as lab safety protocols
Preferred Qualifications:
• Good with technology, in the sense of being able to transfer video, photo, audio files etc. responsibly over OneDrive
• Proficient with video editing applications Adobe Premiere Pro and/or Final Cut Pro (ability to insert opening/closing titles, lower-thirds, Ken Burns effect scroll over photos and videos, background music, etc.)
• Experience with creating closed captioning, preferably in Adobe Premiere Pro
• Knowledge of how to use smartphone and standard cameras and advise on best settings to use
To Apply:
Email PDL Director, Dr. Goltz (nick.goltz@uconn.edu), with any questions and:
– a copy of your CV/resume
– brief statement describing qualifications and interest
– interview availability, and
– contact information for at least 1 professional reference (may be previous employer or instructor)
Interviews will occur on a first-come, first-serve basis until the position is filled. Thank you for your interest!![]()
No Sample Processing April 25th-29th
The UConn Plant Diagnostic Lab will not be able to process samples between April 25th and April 29th. We encourage you to wait to submit samples until Monday, May 2nd. Any samples received between April 25th to April 29th will be logged into our system and kept in cold storage until Dr. Goltz’s return from the NPDN meeting. Anticipate a delayed response if submitting a sample during that window. Any questions regarding plant health or management should be directed to the UConn Home & Garden Education Center (ladybug@uconn.edu). The friendly and helpful staff at the center will support you.

1/4/22 – Lab Accepting Samples, Closed To Visitors
Following updated UConn COVID-19 guidelines, the UConn Plant Diagnostic Lab will be closed to visitors until, at earliest, February 1, 2022. We will be accepting samples as usual during this time, however. If you wish to submit a sample, please either leave the sample in one of the coolers at the wheelchair-accessible entrance of the Ratcliffe Hicks Building (by the Home & Garden Education Center sign), or mail the sample to us, following our sample submission guidelines.
We hope you and your loved ones stay safe, healthy, and warm!
NG
Is your pachysandra suffering? Might be Volutella blight.

During these times of quarantine I’ve become very familiar with my neighborhood sidewalks, using them as new hiking trails. These neighborhood hikes have afforded me a new way to observe a city street landscape, and as a plant pathologist I’ve used this time not only as exercise but to also track common plant disease issues by just keeping my eyes open. You’d be surprised to know how many sick plants are out there to see!
While azaleas, rhododendrons, and Japanese maples are in most neighbors’ yards (and they sure are pretty right now!), I see pachysandra patches nearly everywhere. It is certainly a beloved plant, and very successful as a groundcover. Pachysandra prefers part to full shade and is typically quite low maintenance. The plant naturally grows into a dense carpet, and while this growth pattern usually doesn’t provide any reason for concern, if environmental conditions are right and a particular pathogen is present, disease in a pachysandra patch can spread fairly readily. And that’s exactly what I’ve been seeing this spring.
Volutella blight, caused by the fungus Volutella pachysandricola, is a common destructive disease to pachysandra. The disease presents as brown lesions on the leaves, often with a concentric circle pattern. The lesions expand in size until the whole leaf turns brown-black and dies. Lesions also occur on the stems, causing plants to wither and die back. Orange-pink masses of spores can be seen within lesions during wet and humid conditions. Plants die in patches, and disease spread usually appears in a circular pattern in a bed.
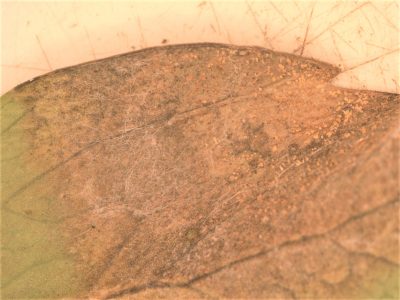
The classic dense planting culture of pachysandra increases the humidity in the bed, providing ideal conditions for Volutella blight to develop and spread. Further, because the pathogen thrives in cool, wet weather, all the rain we’ve had this spring in the Northeast has contributed to disease development as well. Because pachysandra plantings are usually so low maintenance, they can be easy to forget about. As such, stressed plants are more susceptible to Volutella blight. Stress from drought, winter injury, pruning injury, or insect infestations can also contribute to the spread of the disease.
To manage Volutella blight, only work in plants when they are dry, because the fungus moves through water splash. Remove infected plants and destroy them; do not compost as home composting systems typically do not reach high enough temperatures to kill spores. Thin the planting to increase airflow, which also will encourage plants to dry out more quickly. When thinning, sanitize tools with a 10% bleach or 70% rubbing alcohol solution to not accidentally infect new plants. There are fungicide options to help manage Volutella blight, but will only be useful if these other measures are taken. They will not cure infected plants.
So, head outside today and check on your pachysandra patch. Might be time to give it a little love!
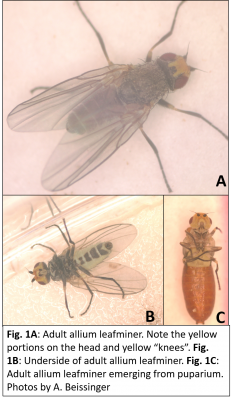
Allium leafminer in Connecticut
Fuzzy raspberries: What causes Botrytis fruit rot?

It is a familiar trope to many of us who buy raspberries in the grocery store. They look fine when you take them off the shelf, give them a rinse at home, and put them in the refrigerator for safe storage. You are excited to put them on your oatmeal, or blend them up in a smoothie. When morning comes, you open the plastic clamshell only to find the berries covered in a gray fuzzy mold. What gives? How did it happen so quickly?
The fuzz you are seeing is Botrytis cinerea, a common fungus that can infect hundreds of species of plants, and raspberries are a particularly preferable host. Botrytis thrives in humid conditions, and can spread extremely quickly. Over 60,000 Botrytis spores can be produced on a single raspberry, and it only takes one spore to cause a new infection. So while you may have rinsed the berries to ensure they were clean and ready to snack on right out of the fridge, those moist conditions were the perfect environment for Botrytis to take hold inside your clamshell.
If you grow raspberries in your own garden, you have more than likely seen Botrytis in your berry patches too. The fungus overwinters as sclerotia, which are black blister-like structures that often protrude from white lesions on canes. During periods of wet weather, spores are produced and disbursed from plant to plant by wind and rain splash. You may see the gray mold colonizing your berries just after the first fruit has ripened. Though, the more overripe the fruit becomes, the more likely the berries will rot and become colonized by Botrytis. If the infection is not managed, serious damage and yield losses can occur on your berry patches. In fact, Botrytis fruit rot is the most common and important disease on raspberries worldwide.
To control the spread of Botrytis in your raspberry (and blackberry) plantings at home, prioritize cultural practices that limit humidity. Ensure your site has good airflow by pruning canes, thinning rows, and controlling weeds. A trellising system can also help train canes upwards, preventing them from falling onto one another. Avoiding excessive nitrogen fertilizer application can help prevent plants from putting out more foliage than necessary.


Good sanitation is also key. Remove overripe fruit, spent flowers, any plant material that appears gray or fuzzy, or canes that have large lesions or black blisters on them. Do not compost infected plant parts, as home compost systems are not able to reach adequate temperatures to kill the fungus. Planting with resistant cultivars, or at the very least, not highly susceptible cultivars, can also help provide some protection.
There are some fungicide applications that can be used for Botrytis, but they will not be effective if the above cultural and sanitation recommendations are not taken. Check with UConn’s Home & Garden Education Center (http://ladybug.uconn.edu) or Plant Diagnostic Laboratory (http://plant.lab.uconn.edu) for more recommendations.
If you still want to buy and eat raspberries from the store, not all hope is lost. You can try one of two options. The first is to buy frozen raspberries, and only take them out of the freezer in a quantity you know you’ll consume in that sitting. Frozen raspberries are great in smoothies and can replace the need for ice. The other option is to take the berries home and store them in the refrigerator with a dry paper towel to help wick away moisture. Then, you can give the fresh berries a rinse just before you’d like to enjoy them. While raspberries may feel like a bit of work to be able to eat them, they sure are tasty!
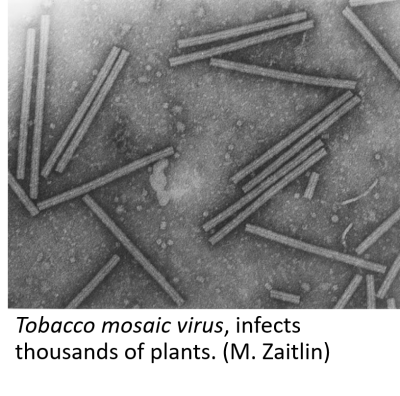
What is a virus?
Diagnostician Out of the Office until 3/13
Our diagnostician will be out of the office to attend the Northeast National Plant Diagnostic Network Meeting and the Northeastern Division-American Phytopathological Society Meeting until 3/12. Diagnoses on samples sent during this time will be delayed, and will resume on 3/13. We appreciate your understanding.
For urgent matters, contact the Home & Garden Education Center (ladybug.uconn.edu) or your UConn IPM Extension Educator (ipm.uconn.edu).


 However, new strains of PVY have evolved to make it more difficult to notice an infection until it is too late to do anything about it. Viruses are capable of evolving to change the symptoms they induce in hosts in order to continue to thrive.
However, new strains of PVY have evolved to make it more difficult to notice an infection until it is too late to do anything about it. Viruses are capable of evolving to change the symptoms they induce in hosts in order to continue to thrive. The other way PVY is spread is through infected seed. When infected seed potatoes are planted, they result in infected plants. These infected plants then are a source of PVY inoculum for aphids.
The other way PVY is spread is through infected seed. When infected seed potatoes are planted, they result in infected plants. These infected plants then are a source of PVY inoculum for aphids.